What Is the Molarity of a Sodium Hydroxide Solution if
Molarity of oxalic acid solution fracM20 Molarity of sodium hydroxide solution m. 3 3 H v PO 4 n 100 If 350 ml of 02 M H2S04 is required to neutralize 250 mf of NaOH.
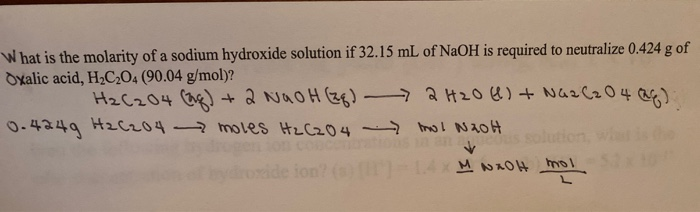
Solved What Is The Molarity Of A Sodium Hydroxide Solution Chegg Com
Molarity refers to the number of moles of the solute present in 1 liter of solution.

. Calculate the molarity of the NaCl solution. M_1V_1moles_2 moles_1moles_2 Since the titration occurred with 25 mL of the 10 solution the moles calculated were only a quarter of all the moles in the whole 100mL. The indicator used Phenolphthalein.
The volume of oxalic acid solution 10cm³. State your solution to the problem. You can also calculate the mass of a substance needed to achieve a desired molarity.
Calculate moles of hydroxide ions nOH-aq. Hydroxide is a diatomic anion with chemical formula OH It consists of an oxygen and hydrogen atom held together by a single covalent bond and carries a negative electric chargeIt is an important but usually minor constituent of waterIt functions as a base a ligand a nucleophile and a catalystThe hydroxide ion forms salts some of which dissociate in aqueous solution. To understand the topic as a whole you will.
Paul Andersen explains pH as the power of hydrogen. It is a white translucent crystalline solid and used in the manufacturing of detergents and soaps. OH-aq 398 10-5 mol L-1.
What is the molarity of sodium hydroxide if 200 ml of the solution is neutralized by 280 ml of 100M I-ICJ solution. To learn about the structure Properties Preparation Uses Health Hazards and FAQs of Sodium hydroxide NaOH. This molarity calculator is a tool for converting the mass concentration of any solution to molar concentration or recalculating grams per ml to moles.
The number of moles of solute and the total volume of the solution is required to compute the molarity of a solution. This article will provide you with the molarity definition and the molarity formula. The pOH of an aqueous solution of sodium hydroxide is 35 Calculate the moles of hydroxide ions in 025 L of this solution.
Concentrated HCl is 121 M. Divide the moles of solute by the litres of the solution to get the molarity. He explains how increases in the hydronium ion or hydrogen ion concentration can lower the pH and crea.
Visit BYJUS for more information. Prelaboratory Assignment 1 Calculate the volume of a concentrated solution of HCl required to prepare a 1 L HCl solution of approximately 01 M. November 3 2002 INTRODUCTION A TITRATION is a process in which a measured amount of a solution is reacted with a known volume of another solution one of the solutions has an unknown concentration until a desired end point is reached.
Think about significant figures. What have you been asked to do. Sodium hydroxide NaOH - Sodium hydroxide is an ionic compound.
Wt solutions of sodium hydroxide dissolved carbon dioxide precipitates as a carbonate. TITRATION OF SULPHURIC ACID WITH SODIUM HYDROXIDE Modified. The molarity of a solution made by dissolving 200 g of NaOH to make a 482 cm 3 solution is 104 M Tips for Solving Concentration Problems In this example the solute sodium hydroxide and solvent water were identified.
To find the molarity of a substance use the following formula. The molecular weight of sodium hydroxide is 40 gmol. Read the lower meniscus of the solution again in the burette and record it as the final burette reading.
Hvo O -voo What is the molarity of sodium hydroxide if 200 ml of the solution is neutralized by 174 ml of 100M H3P04 solution. Repeat this procedure two to three times. Molarity is a unit of concentration measuring the number of moles of a solute per liter of solutionThe strategy for solving molarity problems is fairly simple.
0450 moles of NaCl are dissolved in 950 mL of water. This outlines a straightforward method to calculate the molarity of a solution. Mol What information data have you been given.
The product of molarity and volume of the sodium hydroxide provides the moles of the solution and the moles are equal in the acetic acid when completely titrated. The density of 45 ww Potassium hydroxide solution is 1456 gml at 25C which means that the weight of the 1 ml of Potassium hydroxide solution is 1456 g at 25C. In a 025 molL NaOH solution for example each litre contains 025 mol sodium hydroxide.

How To Calculate The Molarity Of Naoh Solution Moll Chegg Com

Solved Question 15 7 Pts What Is The Molarity Of A Sodium Chegg Com

Solved Part 3 Preparation Of 0 50 M Sodium Hydroxide Solution From Solid Naom Sodium Hydroxide Required Prepare 100 Ml Naoh Olutlon Cilculate The Mas 50 M 409 No Iooml 5 2 0
Comments
Post a Comment